Abstract
Introduction: Cytokine release syndrome (CRS) is a common toxicity associated with chimeric antigen receptor (CAR) T-cell therapies. Corticosteroids and steroid-sparing therapies such as tocilizumab, an interleukin-6 receptor antagonist, and anakinra, an interleukin-1 receptor antagonist, have been used to reduce the incidence and severity of these toxicities. Preclinical and clinical case studies of anakinra, administered subcutaneously or intravenously at various doses, have shown promising results in the management of CRS and systemic inflammatory responses resembling hemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS). In CARTITUDE-1, CRS occurred in 95% of heavily pretreated patients with relapsed/refractory multiple myeloma (RRMM) receiving ciltacabtagene autoleucel (cilta-cel), a CAR T-cell therapy with 2 B-cell maturation antigen-targeting single-domain antibodies (Berdeja et al. Lancet 2021). Per protocol, tocilizumab was required to manage CRS with option to give steroids and/or anakinra per investigator discretion. Here, we report the institutional experiences of anakinra use in the management of CRS in patients who have received cilta-cel as part of the CARTITUDE-1 study.
Methods: Eligible patients had MM and received ≥3 prior therapies or were refractory to a proteasome inhibitor (PI) and immunomodulatory drug (IMiD), and had received a PI, IMiD, and anti-CD38 antibody (Berdeja et al. Lancet 2021). After apheresis, bridging therapy was permitted. Patients received a single cilta-cel infusion (target dose: 0.75×10 6 CAR+ viable T cells/kg; range 0.5-1.0×10 6) 5-7 days after lymphodepletion (300 mg/m 2 cyclophosphamide, 30 mg/m 2 fludarabine daily for 3 days). Lee et al (Blood 2014) grading criteria for CRS were mapped to the ASTCT criteria for CRS. Post-hoc analysis of data revealed use of anakinra at some sites in patients who failed to respond to the initial management of CRS with tocilizumab +/- dexamethasone or in clinical settings where rise of ferritin and/or liver function tests were indicative for continued HLH/MAS-like manifestations (Kennedy et al. ASH 2020).
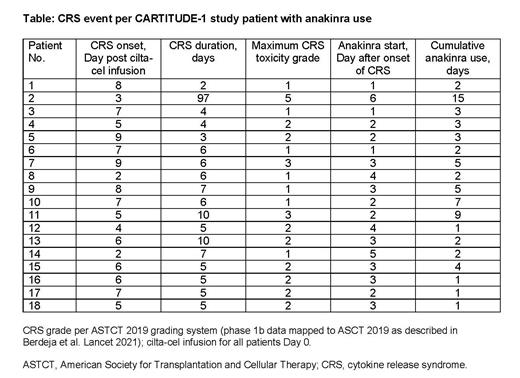
Results: Of 97 patients in CARTITUDE-1, CRS occurred in 92 (95%) patients; 4% were grade 3/4. The median time to onset was 7 days (range 1-12) and median duration was 4 days (range 1-14). Supportive measures to treat CRS were administered to 91% of patients, most commonly tocilizumab (69%; 4 patients received ≥3 doses), corticosteroids (22%), and anakinra (18 patients, 19%). CRS resolved in 99% of patients. Anakinra was administered after initial tocilizumab and within the first 48 hours (range 0-6 days) of CRS onset for the majority of patients as part of effective management of CRS. Anakinra was administered at a dose of 100-200 mg every 8-12 hours over a median of 2.5 days (range 1-15 days). CRS uniformly resolved following anakinra use in CARTITUDE-1, apart from one patient who died from sepsis (grade 5 outcome) due to HLH/MAS considered related to treatment (Table).
Conclusions: CRS events in cilta-cel-treated patients in CARTITUDE-1 were common, generally low-grade, and successfully managed with standard tocilizumab +/- dexamethasone. The use of anakinra should be considered in patients with persistent CRS/inflammatory symptoms despite tocilizumab use, and in particular in patients with HLH/MAS-like symptoms/phenotype occurring following CRS or in the absence of prior CRS.
Wong: Amgen: Consultancy; Genentech: Research Funding; Fortis: Research Funding; Janssen: Research Funding; GloxoSmithKlein: Research Funding; Dren Biosciences: Consultancy; Caelum: Research Funding; BMS: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. Richard: Karyopharm, Janssen: Honoraria. Lin: Juno: Consultancy; Legend: Consultancy; Merck: Research Funding; Bluebird Bio: Consultancy, Research Funding; Sorrento: Consultancy; Janssen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Novartis: Consultancy; Celgene: Consultancy, Research Funding; Takeda: Research Funding; Gamida Cell: Consultancy; Vineti: Consultancy. Madduri: Janssen: Current Employment. Jackson: Janssen: Current Employment; Memorial Sloan Kettering Cancer Center: Consultancy. Zudaire: Janssen: Current Employment. Romanov: Janssen: Current Employment. Trigg: Janssen: Current Employment. Vogel: Janssen Global Services, LLC: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Garrett: Legend Biotech USA: Current Employment. Nesheiwat: Legend Biotech USA: Current Employment. Martin: Oncopeptides: Consultancy; Sanofi: Research Funding; Janssen: Research Funding; GlaxoSmithKline: Consultancy; Amgen: Research Funding. Jagannath: Bristol Myers Squibb: Consultancy; Legend Biotech: Consultancy; Karyopharm Therapeutics: Consultancy; Janssen Pharmaceuticals: Consultancy; Takeda: Consultancy; Sanofi: Consultancy.
At the time of abstract submission, cilta-cel is being investigated for the treatment of multiple myeloma but is not yet approved

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal